PARTICIPANTS NEEDED FOR
RESEARCH INTO NON-SURGICAL VOLUMIZATION OF TEMPORAL HOLLOWS / CHEEK AUGMENTATION
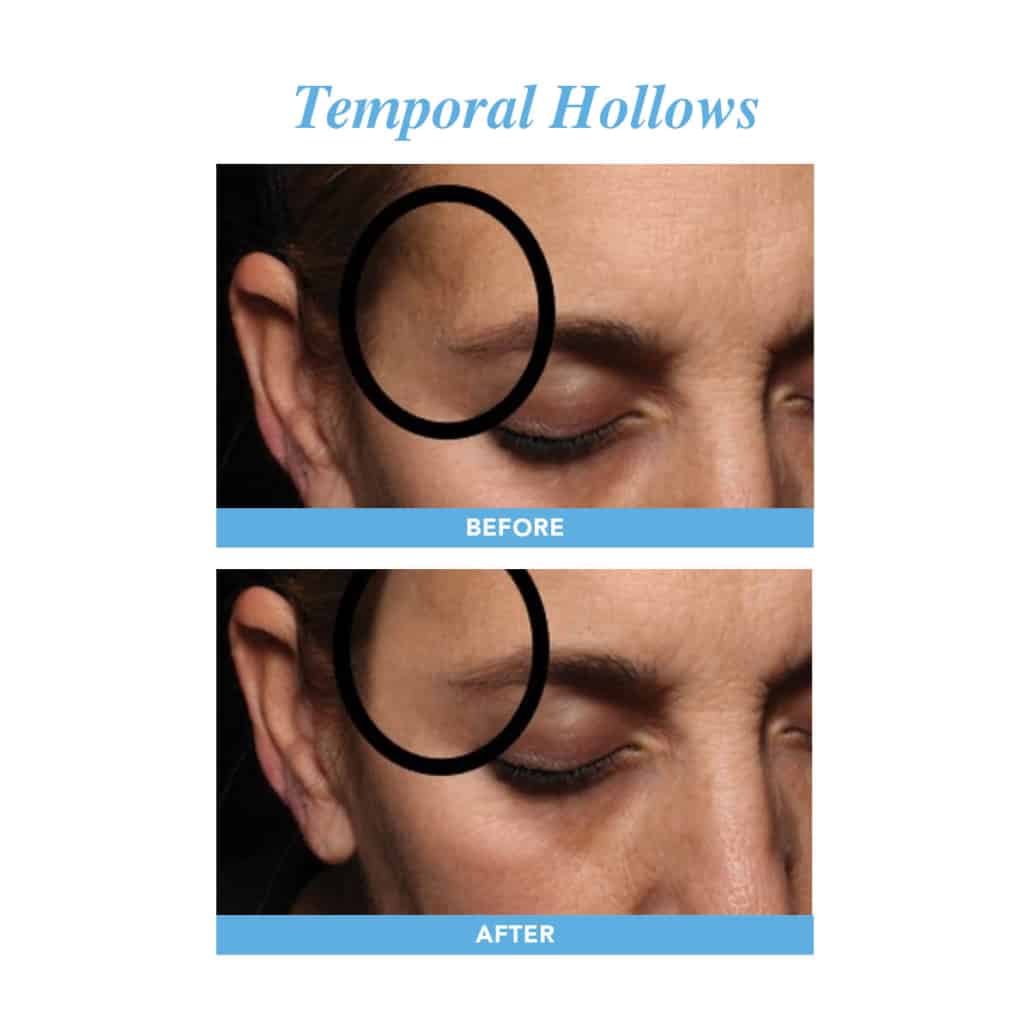
We are looking for female volunteers to take part in a study regarding the use of hyaluronic acid injections in the temporal and/ or cheek regions. These treatments are used to improve facial appearance, by replacing volume loss and correcting the contour.
With age, patients lose facial volume. This can create a “skeleton” appearance in the temporal region or contour deficits in the midface (cheek) region.
The aim of the current study is to compare two family of fillers, including aesthetic outcome, injection volume, and longevity.
The products used in this study are fully approved for sale and use in Canada. In this study, the study products will only be administered by a board-certified plastic surgeon (Dr. Andreas Nikolis).
IMPORTANT INFO FOR PARTICIPANTS
The study participation will last for 8 weeks (2 months) from the date of injection.
Each subject that successfully completes all study related procedures will be seen over 5 visits:
-
- Visit 1: Screening/Baseline (Treatment; Week 0)
- Visit 2: Follow-up 1 (Week 1)
- Visit 3: Follow-up 2 (Week 4)
- Visit 4: Follow-up 3 (Week 6)
- Visit 5: Follow-up 4 (Week 8)
You must provide photo consent for research, educational and marketing purposes.